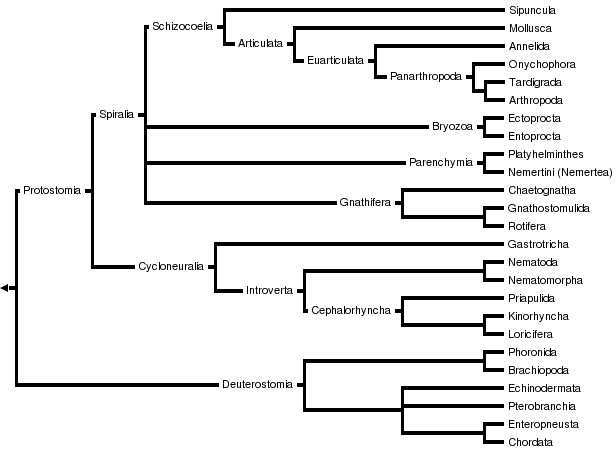
Bilateria
Triploblasts, Bilaterally symmetrical animals with three germ layers
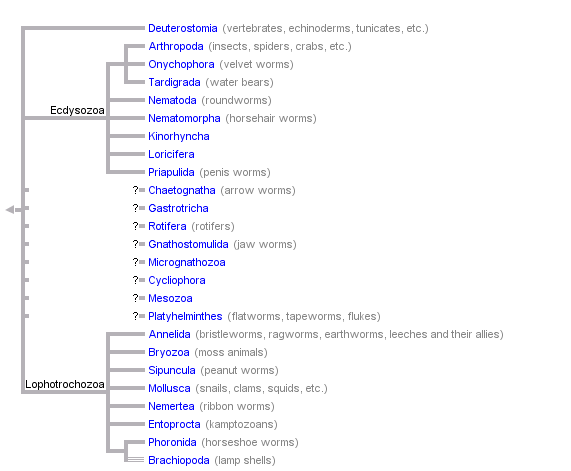

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxThis tree represents the view of bilaterian relationships as it is currently emerging from analyses based on molecular data (mostly 18S rRNA sequences). For an alternative hypothesis of bilaterian relationships based on morphological data, see the Discussion of Phylogenetic Relationships.
Discussion of Phylogenetic Relationships
Due to new evidence from developmental biology and molecular phylogenetics, ideas about bilaterian relationships have undergone a major paradigm shift within the last decade. The new hypotheses shown in the tree above are now widely accepted, but there are also many sceptics who emphasize the pitfalls and inconsistencies associated with the new data. One of the most prominent alternative views based on morphological evidence is championed by Nielsen (2001):
References
Anderson, F. E., A. J. Cordoba, and M. Thollesson. 2004. Bilaterian phylogeny based on analyses of a region of the sodium-potassium ATPase alpha-subunit gene. Journal of Molecular Evolution 58:252-268.
Adoutte, A., G. Balavoine, N. Lartillot, and R. de Rosa. 1999. Animal evolution, the end of the intermediate taxa? Trends in Genetics 15:104-108.
Aguinaldo, A. M. A., J. M. Turbeville, L. S. Linford, M. C. Rivera, J. R. Garey, R. A. Raff, and J. A. Lake. 1997. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387:489-493.
Baguñà, J., P. Martinez, J. Paps, and M. Riutort. 2008. Back in time: a new systematic proposal for the Bilateria. Philosophical Transactions of the Royal Society Series B 363(1496):1481-1491.
Balavoine, G. 1997. The early emergence of platyhelminths is contradicted by the agreement between 18S rRNA and Hox genes data. C. R. Acad. Sci. Paris, Sciences de la vie/Life Sciences 320:83-94.
Balavoine, G. 1998. Are Platyhelminthes coelomates without a coelom? An argument based on the evolution of Hox genes. American Zoologist 38:843-858.
Balavoine, G. and A. Adoutte. 1998. One or three cambrian radiations? Science 280:397-398
Balavoine, G., R. de Rosa, and A. Adoutte. 2002. Hox clusters and bilaterian phylogeny. Molecular Phylogenetics and Evolution 24:366-373.
Boore, J. L. and W. M. Brown. 2000. Mitochondrial genomes of Galathealinum, Helobdella, and Platynereis: Sequence and gene arrangement comparisons indicate that Pogonophora is not a phylum and Annelida and Arthropoda are not sister taxa. Molecular Biology and Evolution 17:87-106.
Boore, J. L. and J. L. Stanton. 2002. The mitochondrial genome of the sipunculid Phascolopsis gouldii supports its association with Annelida rather than Mollusca. Molecular Biology and Evolution 19:127-137.
Bourlat, S. J., C. Nielsen, A. D. Economoua, and M. J. Telford. 2008. Testing the new animal phylogeny: A phylum level molecular analysis of the animal kingdom. Molecular Phylogenetics and Evolution 49(1):23-31.
Bourlat, S., C. Nielsen, A. Lockyer, D. T. Littlewood, and M. Telford. 2003. Xenoturbella is a deuterostome that eats molluscs. Nature 424:925-928.
Budd, G. E. and S. Jensen. 2000. A critical reappraisal of the fossil record of the bilaterian phyla. Biol Rev 75: 253–295.
Carranza, S., J. Baguña, and M. Riutort. 1997. Are the platyhelminthes a monophyletic primitive group? An assessment using 18S rDNA sequences. Molecular Biology and Evolution 14:485-497.
Copley, R. R. 2008. The animal in the genome: comparative genomics and evolution. Philosophical Transactions of the Royal Society Series B 363(1496):1453-1461.
Denes, A., G. Jékely, P. Steinmetz, F. Raible, H. Snyman, B. Prud’homme, D. Ferrier, G. Balavoine, D. Arendt. 2007. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell 129(2):277-288.
deRosa R. 2001. Molecular data indicate the protostome affinity of brachiopods. Systematic Biology 50:848-859.
deRosa R., J. K. Grenier, T. Andreeva, C. E. Cook, A. Adoutte, M. Akam, S. B. Carroll, and G. Balavoine. 1999. Hox genes in brachiopods and priapulids and protostome evolution. Nature 399:772-776.
Dewel, R. A. and W. C. Dewel. 1998. The place of tardigrades in arthropod evolution. Pages 109-123 in Arthropod Relationships (R.A. Fortey and R.H. Thomas, eds.) The Systematics Association and Chapman and Hall, London.
Dopazo, H. and J. Dopazo. 2005. Genome-scale evidence of the nematode-arthropod clade. Genome Biol 6: R41.
Dunn, C. W., A Hejnol, D. Q. Matus, K. Pang, W. E. Browne, S. A. Smith, E. Seaver, G. W. Rouse, M. Obst, G. D. Edgecombe, M. V. Sørensen, S. H. D. Haddock, A. Schmidt-Rhaesa, A. Okusu, R. M. Kristensen, W. C. Wheeler, M. Q. Martindale, and G. Giribet. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. doi:10.1038/nature06614
Eernisse, D. J. 1998. Arthropod and annelid relationships re-examined. Pages 43-56 in Arthropod Relationships (R.A. Fortey and R.H. Thomas, eds.) The Systematics Association and Chapman and Hall, London.
Eernisse, D. J., J. S. Albert, F. E. Anderson. 1992. Annelida and Arthropoda are not sister taxa. A phylogenetic analysis of spiralian metazoan morphology. Systematic Biology 41:305-330.
Erwin, D. H. and E. H. Davidson. 2002. The last common bilterian ancestor. Development 129:3021-32.
Funch, P., M.V. Sørensen and M. Obst. 2005. On the phylogenetic position of Rotifera—have we come any further? Hydrobiologia 546:11–28.
Garey, J. R. 2001. Ecdysozoa: The relationship between Cycloneuralia and Panarthropoda. Zoologischer Anzeiger 240: 321-330.
Garey, J. R., M. Krotec, D. R. Nelson, and J. Brooks. 1996. Molecular analysis supports a tardigrade-arthropod association. Invertebrate Biology 115:79-88.
Garey, J. R. and A. Schmidt-Rhaesa. 1998. The essential role of "minor" phyla in molecular studies of animal evolution. American Zoologist 38:907-917.
Giribet, G. 2003. Molecules, development and fossils in the study of metazoan evolution; Articulata versus Ecdysozoa revisited. Zoology 106:303-326.
Giribet, G., S. Carranza, J. Baguna, M. Riutort and C. Ribera. 1996. First molecular evidence for the existence of a Tardigrada plus arthropoda clade. Molecular Biology and Evolution 13:76-84.
Giribet, G., D. L. Distel, M. Polz, W. Sterrer and W. Wheeler. 2000. Triploblastic relationships with emphasis on the acoelomates and the position of Gnathostomulida, Cycliophora, Plathelminthes, and Chaetognatha: a combined approach of 18S rDNA sequences and morphology. Systematic Biology 49:539-562.
Giribet, G., C. W. Dunn, G. D. Edgecombe, and G. W. Rouse. 2007. A modern look at the Animal Tree of Life. Pages 61-79 in: Zhang, Z.-Q. & Shear, W.A., eds. Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa 1668:1-766.
Giribet, G. and C. Ribera. 1998. The position of arthropods in the animal kingdom: A search for a reliable outgroup for internal arthropod phylogeny. Molecular Phylogenetics and Evolution 9:481-488.
Giribet, G., M. V. Sørensen, P. Funch, R. M. Kristensen, and W. Sterrer. 2004. Investigations into the phylogenetic position of Micrognathozoa using four molecular loci. Cladistics 20:1-13.
Giribet G. and W. C. Wheeler. 1999. The position of arthropods in the animal kingdom: Ecdysozoa, islands, trees, and the "parsimony ratchet". Molecular Phylogenetics and Evolution 13:619-623.
Halanych, K. M., J. D. Bacheller, A. M. A. Aguinaldo, S. M. Liva, D. M. Hillis, and J. A. Lake. 1995. Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science 267: 1641-1643.
Halanych, K. 2004. The new view of animal phylogeny. Annual Review of Ecology, Evolution, and Systematics 35:229-256.
Hanelt, B., D. VanSchyndel, C. M. Adema, L. A. Lewis, E. S. Loker. 1996. The phylogenetic position of Rhopalura ophiocomae (Orthonectida) based on 18S ribosomal DNA sequence analysis. Molecular Biology and Evolution 13:1187-1191.
Hausdorf, B., M. Helmkampf, A. Meyer, A. Witek, H. Herlyn, I. Bruchhaus, T. Hankeln, T. H. Struck, and B. Lieb. 2007. Spiralian phylogenomics supports the resurrection of Bryozoa comprising Ectoprocta and Entoprocta. Molecular Biology and Evolution 24(12):2723-2729.
Hejnol, A. and M. Q. Martindale. 2008. Acoel development supports a simple planula-like urbilaterian. Philosophical Transactions of the Royal Society Series B 363(1496):1493-1501.
Helfenbein, K. G. and J. L. Boore. 2004. The mitochondrial genome of Phoronis architecta comparisons demonstrate that phoronids are lophotrochozoan protostomes. Molecular Biology and Evolution 21:153-57.
Helfenbein, K. G., H. M. Fourcade, R. G. Vanjani, and J. L. Boore. 2004. The mitochondrial genome of Paraspadella gotoi is highly reduced and reveals that chaetognaths are a sister group to protosotomes. Proceedings of the National Academy of Sciences (USA) 101:10639-43.
Helmkampf, M., I. Bruchhaus and B. Hausdorf. 2008. Multigene analysis of lophophorate and chaetognath phylogenetic relationships. Molecular Phylogenetics and Evolution 46(1):206-214.
Irimia, M., I. Maeso, D. Penny, J. Garcia-Fernandez, and S. W. Roy. 2007. Rare coding sequence changes are consistent with Ecdysozoa, not Coelomata. Molecular Biology and Evolution 24:1604-1607.
Jenner, R. A. 2001. Bilaterian phylogeny and uncritical recycling of morphological data sets. Systematic Biology 50:730-742.
Jenner, R. A. 2004. Towards a phylogeny of the Metazoa: evaluating alternative phylogenetic positions of Platyhelminthes, Nemertea, and Gnathostomulida, with a critical reappraisal of cladistic characters. Contributions to Zoology 73 (1-2):3-163.
Jenner, R. A. and D. T. J. Littlewood. 2008. Problematica old and new. Philosophical Transactions of the Royal Society Series B 363(1496):1503-1512.
Jenner, R. A. and F. R. Schram. 1999. The grand game of metazoan phylogeny: rules and strategies. Biological Reviews 74:121-142.
Katayama, T., H. Wada, H. Furuya, N. Satoh, and M. Yamamoto. 1995. Phylogenetic position of the dycyemid Mesozoa inferred from 18S rDNA sequences. Biological Bulletin 189:81-90.
Kobayashi, M., H. Furuya, and P. W. H. Holland. 1999. Dicyemids are higher animals. Nature 401:762.
Lartillot, N. and H. Philippe. 2008. TImprovement of molecular phylogenetic inference and the phylogeny of Bilateria. Philosophical Transactions of the Royal Society Series B 363(1496):1463-1472.
Littlewood, D. T. J., M. J. Telford, K. A. Clough, K. Rohde. 1998. Gnathostomulida - an enigmatic metazoan phylum from both morphological and molecular perspectives. Molecular Phylogenetics and Evolution 9:72-79.
Lüter, C. 1997. The phylogenetic position of Brachiopoda - a comparison of morphological and molecular data. Zoologica Scripta 26:245-253.
Mackey, L. Y., B. Winnepenninckx, R. DeWachter, T. Backeljau, P. Emschermann, and J. R. Garey. 1996. 18S rRNA suggests that Entoprocta are protostomes, unrelated to Ectoprocta. Journal of Molecular Evolution 42:552-559.
Mallatt, J., J. R. Garey, and J. W. Shultz. 2004. Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Molecular Phylogenetics and Evolution (31)1:178-191.
Mallatt, J. and G. Giribet. 2006. Further use of nearly complete 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Molecular Phylogenetics and Evolution 40(3):772-794.
Mallatt, J. and C. J. Winchell. 2002. Testing the new animal phylogeny: First use of combined large-subunit and small-subunit rRNA gene sequences to classify the protostomes. Molecular Biology and Evolution 19:289-301.
Manuel, M., M. Kruse, W. E. G. Müller, and Y. L. Parco. 2000. The comparison of -thymosin homologues among Metazoa supports an arthropod-nematode clade. Journal of Molecular Evolution 51:378-81.
Marletaz, F., E. Martin, Y. Perez, D. Papillon, X. Caubit, C. Lowe, B. Freeman, L. Fasano, C. Dossat, P. Wincker. 2006. Chaetognath phylogenomics: A protostome with deuterostome-like development. Current Biology 16: R577–578.
Matus, D. Q., R. R. Copley, C. W. Dunn, A. Hejnol, H. Eccleston, K. M. Halanych, M. Q. Martindale, and M. J. Telford. 2006. Broad taxon and gene sampling indicate that chaetognaths are protostomes. Current Biology 16:R575–576.
McHugh, D. and G. W. Rouse. 1998. Life history evolution of marine invertebrates: new views from phylogenetic systematics. Trends in Ecology and Evolution 13: 182-186.
Minelli, A. 2007. Invertebrate taxonomy and evolutionary developmental biology. Pages 55-60 in: Zhang, Z.-Q. & Shear, W.A., eds. Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa 1668:1-766.
Nielsen, C. 1998. The phylogenetic position of the Arthropoda. Pages 11-22 in Arthropod Relationships (R. A. Fortey and R. H. Thomas, eds.) Systematics Association Special Volume Series 55. Chapman & Hall, London.
Nielsen, C. 2001. Animal Evolution: Interrelationships of the Living Phyla. Second Edition. Oxford University Press, Oxford.
Nielsen, C. 2003. Proposing a solution to the Articulata-Ecdysozoa controversy. Zoologica Scripta 32:475-82.
Nielsen, C., N. Scharff, and D. Eibye-Jacobsen. 1996. Cladistic analyses of the animal kingdom. Biological Journal of the Linnean Society 57:385-410.
Papillon, D., Y. Perez, X. Caubit, and Y. Le Parco. 2004. Identification of chaetognaths as protostomes is supported by the analysis of their mitochondrial genome. Molecular Biology and Evolution 21(11):2122-2129.
Passamaneck, Y. J. and K. M. Halanych. 2004. Evidence from Hox genes that bryozoans are lophotrochozoans. Evol. Dev. 6:275-281.
Passamaneck, Y. J. and K. M. Halanych. 2006. Lophotrochozoan phylogeny assessed with LSU and SSU data: Evidence of lophophorate polyphyly. Molecular Phylogenetics and Evolution 40(1):20-28.
Pawlowski, J., J. I. MontoyaBurgos, J. F. Fahrni, J. Wuest, and L. Zaninetti. 1996. Origin of the Mesozoa inferred from 18S rRNA gene sequences. Molecular Biology and Evolution 13:1128-1132.
Peterson, K. J., R. A. Cameron and E. H. Davidson. 2000. Bilaterian origins: Significance of new experimental observations. Developmental Biology 219:1-17.
Peterson, K. J., J. A. Cotton, J. G. Gehling and D. Pisani. 2008. The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records. Philosophical Transactions of the Royal Society Series B 363(1496):1435-1443.
Peterson, K. J. and D. J. Eernisse. 2001. Animal phylogeny and the ancestry of bilaterians: inferences from morphology and 18S rDNA gene sequences. Evolution & Development 3:170-205.
Philip, G. K., C. J. Creevey, and J. O. McInerney. 2005. The Opisthokonta and the Ecdysozoa may not be clades: Stronger support for the grouping of plant and animal than for animal and fungi and stronger support for the Coelomata than Ecdysozoa. Molecular Biology and Evolution 22(5):1175–1184.
Philippe, H., N. Lartillot, and H. Brinkmann. 2005. Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Molecular Biology and Evolution 22(5):1246-1253.
Podsiadlowski, L., A. Braband, and G. Mayer. 2008. The complete mitochondrial genome of the onychophoran Epiperipatus biolleyi reveals a unique transfer RNA set and provides further support for the Ecdysozoa hypothesis. Molecular Biology and Evolution 25(1):42-51. doi:10.1093/molbev/msm223
Raff, R. A. 2008. Origins of the other metazoan body plans: the evolution of larval forms. Philosophical Transactions of the Royal Society Series B 363(1496):1473-1479.
Rogozin, I. B., Y. I. Wolf, L. Carmel and E. V. Koonin. 2007. Ecdysozoan clade rejected by genome-wide analysis of rare amino acid replacements. Molecular Biology and Evolution 24(4):1080-1090. doi:10.1093/molbev/msm029
Rogozin, I. B., Y. I. Wolf, L. Carmel and E. V. Koonin. 2007. Analysis of rare amino acid replacements supports the Coelomata clade. Molecular Biology and Evolution 24(12):2594-2597.
Rokas, A., D. Kruger, and S. B. Carroll. 2005. Animal evolution and the molecular signature of radiations compressed in time. Science 310:1933–1938.
Roy, S. W. and M. Irimia. 2008. Rare genomic characters do not support Coelomata: Intron loss/gain. Molecular Biology and Evolution 25:620-623.
Ruiz-Trillo, I., J. Paps, M. Loukota, C. Ribera, U. Jondelius, J. Baguña, and M. Riutort. 2002. A phylogenetic analysis of myosin heavy chain type II sequences corroborates that Acoela and Nemertodermatida are basal bilaterians. Proceedings of the Natlional Academy of Sciences (USA) 99:11246-11251.
Ruiz-Trillo, I., M. Riutort, D. T. J. Littlewood, E. A. Herniou, J. Baguña. 1999. Acoel flatworms: earliest extant bilaterian metzoans, not members of platyhelminthes. Science 283:1919-1923.
Schmidt-Rhaesa, A., T. Bartolomaeus, C. Lemburg, U. Ehlers, and J. R. Garey. 1998. The position of the Arthropoda in the phylogenetic system. Journal of Morphology 238:263-285.
Schmidt-Rhaesa, A. 1998. Phylogenetic relationships of the nematomorpha - a discussion of current hypotheses. Zoologischer Anzeiger 236:203-216.
Scholtz, G., 2002: The Articulata hypothesis - or what is a segment? Organisms Diversity & Evolution 2:197-215.
Seaver, E. C. 2003. Segmentation: mono- or polyphyletic? Int. J. Dev. Biol. 47:583-595.
Sørensen, M. V., P. Funch, E. Willerslev, A. J. Hansen, and J. Olesen. 2000. On the phylogeny of the Metazoa in light of Cycliophora and Micrognathozoa. Zoologischer Anzeiger 239:297-318.
Sørensen, M. V., M. B. Hebsgaard, I. Heiner, H. Glenner, E. Willerslev, and R. M. Kristensen. 2008. New data from an enigmatic phylum: evidence from molecular sequence data supports a sister-group relationship between Loricifera and Nematomorpha. Journal of Zoological Systematics and Evolutionary Research 46(3):231-239.
Stechmann, A. and M. Schlegel. 1999. Analysis of the complete mitochondrial DNA sequence of the brachiopod Terebratulina retusa places Brachiopoda within the protostomes. Procedings of the Royal Society London Series B 266:2043-2052.
Struck, T. H. and F. Fisse. 2008. Phylogenetic position of Nemertea derived from phylogenomic data. Molecular Biology and Evolution 25:728-736.
Telford, M. J. 2007. A single origin of the central nervous system? Cell 129(2):237-239.
Telford, M. J. and D. T. Littlewood. 2008. The evolution of the animals: introduction to a Linnean tercentenary celebration. Philosophical Transactions of the Royal Society Series B 363(1496):1421-1424.
Telford, M. J., A. E. Lockyer, C. Cartwright-Finch, and D. T. Littlewood. 2003. Combined large and small subunit ribosomal RNA phylogenies support a basal position of the acoelomorph flatworms. Proc. R. Soc. London Ser. B 270:1077-83.
Turbeville, J. M., K. G. Field, and R. A. Raff. 1992. Phylogenetic position of phylum Nemertini, inferred from 18s rRNA sequences: Molecular data as a test of morphological character homology. Molecular Biology and Evolution 9:235-249.
Valentine, J. W. 1994. Late Precambrian bilaterians: grades and clades. Proceedings of the National Academy of Sciences (U.S.A.) 91:6751-6757.
Valentine, J. W. 1997. Cleavage patterns and the topology of the metazoan tree of life. Proceedings of the National Academy of Sciences (U.S.A.) 94:8001-8005.
Valentine, J. W. and A. G. Collins. 2000. The significance of moulting in Ecdysozoan evolution. Evolution and Devlopment 2(3):152-156.
von Nickisch-Rosenegk, M., W. M. Brown, and J. L. Boore. 2001. Complete sequence of the mitochondrial genome of the tapeworm Hymenolepis diminuta: Gene arrangements indicate that platyhelminths are eutrochozoans. Molecular Biology and Evolution 18:721-730.
Wada, H., and N. Satoh. 1994. Details of the evolutionary history from invertebrates to vertebrates, as deduced from the sequences of 18S rDNA. Proceedings of the National Academy of Sciences (USA) 91:1801-1804.
Waeschenbach, A., M. J. Telford, J. S. Porter, and D. T. J. Littlewood. 2006. The complete mitochondrial genome of Flustrellidra hispida and the phylogenetic position of Bryozoa among the Metazoa. Molecular Phylogenetics and Evolution 40(1):195-207.
Waggoner, B. M. 1998. Interpreting the earliest metazoan fossils: What can we learn? American Zoologist 38:975-982.
Winnepenninckx, B., T. Backeljau, and R. M. Kristensen. 1998. Relations of the new phylum Cycliophora. Nature 393:636-638.
Winnepenninckx, B., T. Backeljau, L. Y. Mackey, J. M. Brooks, R. de Wachter, S. Kumar, and J. R. Garey. 1995. 18S rRNA data indicate that aschelminthes are polyphyletic in origin and consist of at least three distinct clades. Molecular Biology and Evolution 12:1132-1137.
Winnepenninckx, B., T. Backeljau, and R. de Wachter. 1995. Phylogeny of protostome worms derived from 18S rRNA sequences. Molecular Biology and Evolution 12:641-649.
Winnepenninckx, B., Y. van de Peer, and T. Backeljau. 1998. Metazoan relationships on the basis of 18S rRNA sequences: a few years later... American Zoologist 38:888-906.
Wolf, Y. I., I. B. Rogozin, and E. V. Koonin. 2004. Coelomata and not Ecdysozoa: Evidence from genome-wide phylogenetic analysis. Genome Research 14:29–36.
Title Illustrations

| Location | Sacha Lodge, Oriente, Ecuador |
|---|---|
| Specimen Condition | Live Specimen |
| Source | Hoatzin |
| Source Collection | Flickr |
| Copyright |
© 2007 Jim Scarff

|
| Scientific Name | Pisaster ochraceus |
|---|---|
| Location | Protection Island, British Columbia |
| Specimen Condition | Live Specimen |
| Source | Purple Starfish |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0.
|
| Copyright | © 2006 Alanna@VanIsle |
| Scientific Name | Argiope |
|---|---|
| Location | Dallas, Texas |
| Comments | Argiope spider (Arthropoda) on web with stabilimentum |
| Copyright |
© Greg and Marybeth Dimijian

|
| Scientific Name | Cyphoma gibbosum |
|---|---|
| Location | Curacao, Netherland Antilles |
| Specimen Condition | Live Specimen |
| Source | Flamingo Tongue Snail in Profile |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution License - Version 2.0. This media file is licensed under the Creative Commons Attribution License - Version 2.0.
|
| Copyright | © 2006 LASZLO ILYES |
| Scientific Name | Bipalium |
|---|---|
| Location | Dallas, Texas |
| Comments | A planarian flatworm (Platyhelminthes) after emerging from wet soil after rain |
| Copyright |
© Greg and Marybeth Dimijian

|
About This Page
Page copyright © 2002
 Page: Tree of Life
Bilateria. Triploblasts, Bilaterally symmetrical animals with three germ layers.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Bilateria. Triploblasts, Bilaterally symmetrical animals with three germ layers.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Citing this page:
Tree of Life Web Project. 2002. Bilateria. Triploblasts, Bilaterally symmetrical animals with three germ layers. Version 01 January 2002 (temporary). http://tolweb.org/Bilateria/2459/2002.01.01 in The Tree of Life Web Project, http://tolweb.org/














 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site