Dendrophylliina
Dendrophylliidae
Stephen D. Cairns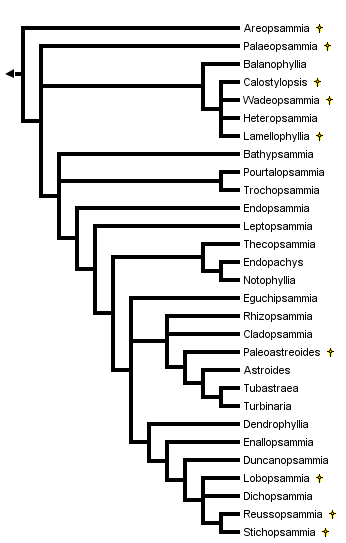

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Dendrophylliids are known from the Early Cretaceous (about 120 million years ago) to the Recent, and are widespread in today's oceans from the North Sea to the Subantarctic, ranging in depths from 0 to 2165 meters. Because most dendrophylliid genera and species do not rely on unicellular, photosyntethic zooxanthellae symbionts, they can live in deep-water environments or cryptic (caves or under ledges) shallow-water habitats that do not receive light. Only a few genera (Turbinaria and Duncanopsammia, and some species of Heteropsammia) contain zooxanthellae in their polyps and consequently manufacture large skeletons that contribute to shallow water reef structure. The remaining genera and species (approximately 149 of the 166 Recent species, 90%) are azooxanthellate. There are an additional 198 exclusively fossil species (Cairns, 2001). The azooxanthellate dendrophylliids range in size from small solitary, interstitial coralla only 5 mm in maximum diameter (e.g., Notophyllia) to large colonies up to a meter in diameter (e.g., Enallopsammia) that, together with similar colonies, contribute to the framework of deep-water coral banks found at depths of 600-800 m in the Straits of Florida (Cairns and Stanley, 1982).

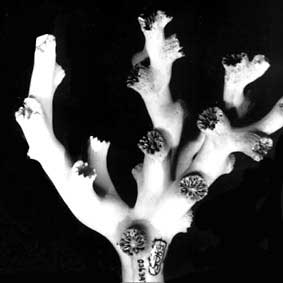
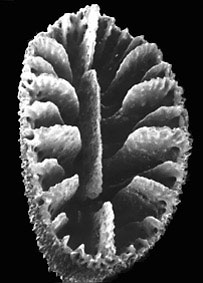
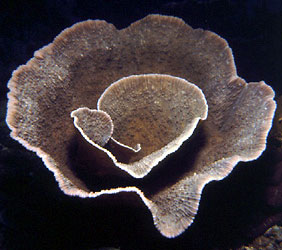
Left (top): Dendrophyllia oldroydae: Holotypic colony, collected from off California (depth 366 m). This species is an example of a colonial, azooxanthellate dendrophylliid. Colony 83 mm in height (from Cairns, 1994). Right (bottom): Notophyllia recta: Calicular view (SEM) of a skeleton collected off Victoria, Australia (depth 110 m), exemplifying a solitary, unattached dendrophylliid. Greater calicular diameter 5 mm. Photograph from Cairns and Parker, 1992. Copyright © 1992 South Australian Museum.
As stated above, dendrophylliids occur as solitary polyps or as colonies of interconnected polyps. Most colonial dendrophylliids are firmly attached to a substrate, probably in order to stabilize their larger mass, but solitary coralla may be attached or free, some on or in a sandy substrate. Two genera (Heteropsammia and Wadeopsammia) have an obligatory symbiosis with a sipunculid worm (Yonge, 1975), which burrows into the base of the solitary corallum and moves the coral from place to place. Some genera (i.e., Endopachys, Eguchipsammia, Balanophyllia) are known to asexually bud smaller coralla from their edges, the parent corallum continuing to increase in size, and one genus (Notophyllia) asexually propagates by transverse division. Six genera are hosts for the galls of ascothoracidan Crustacea (Grygier and Zibrowius, 1985), and two genera are hosts for burrows of acrothoracican cirripeds (Grygier and Newman, 1985; Cairns and Zibrowius, 1997).
Although dendrophylliids are common in the marine environment, they are inconspicuous and therefore are not well known and have not received many common names. However, many of the scientific names of the genera end in the suffix "-psammia", from the Greek psammos (meaning sand), which is an allusion to the fact that the skeletal porosity of all dendrophylliids confers a rough, sandy texture to the touch, like that of fine sandpaper.
Characteristics
The most distinctive feature of the dendrophylliids is their irregularly porous thecal wall, called a synapticulotheca, the pores and tissue lining the pores being continuous from the outside to the inside of the corallum. Some genera also have a thin epitheca that overlays part or all of the synapticulotheca. The septa are also sometimes porous. Otherwise, dendrophylliids are similar to the caryophylliids, having lamellar, smooth-edged septa (minitrabeculate), and a variety of columellar and palar structures. The septa of many, but not all, dendrophylliid genera are arranged according to the so-called Pourtalès plan (Text-Figure), which is not found in any other family. This arrangement results in septa of higher cycles being longer and fusing in front of septa of lower cycles in a distinctive pattern (see Wells, 1956: fig. 239).

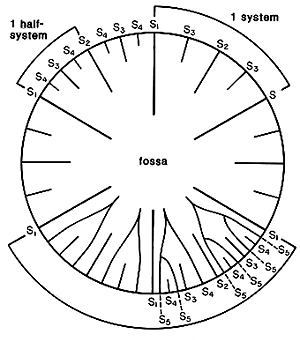

Left (top): Composite cross-sectional diagram of a calice illustrating various septal insertion patterns: upper right system with three cycles of septa, upper left system with four cycles, and lower two systems with various stages of development of the Poutralès plan. Numbers refer to cycle to which septa belong (from Cairns, 1994). Right (bottom): Balanophyllia malouinensis: Calicular view of a skeleton collected from Tierra del Fuego (depth 512 m), illustrating the Pourtalès plan of septal arrangement. This species is an example of a solitary, attached dendrophylliid. Greater calicular diameter 12 mm. Copyright © 2002 S. D. Cairns.
Other papers containing significant information on living dendrophylliids include: Cairns (1977, 2001), van der Horst (1922, 1927), Marshall (1996), Veron and Pichon (1980), and Zibrowius (1973, 1980).
Discussion of Phylogenetic Relationships
A phylogenetic analysis of the 29 dendrophylliid genera was performed by Cairns (2001) based exclusively on gross morphological characters. The tree does not support the separation of the family into two subfamilies, but does aggregate those genera having a solitary corallum from those that are colonial. Furthermore, the colonial genera are divided into three clades: one having quasicolonial branching; a second having arborescent branching; and a third having non-arborescent colonies. Cairns (2001) considered this to be a poorly supported phylogenetic hypothesis and recommended farther study using characteristics of the soft parts and molecular sequencing, when possible.
The monophyly of this family is well supported by 16S mtDNA sequences (Romano and Cairns, 2000), which unites all six dendrophylliid genera tested as a clade. Other published molecular analyses also place the dendrophylliid genera in a separate clade (Romano and Palumbi, 1996; Veron, et al., 1996).
References
Cairns, S. D. 1977. A review of the Recent species of Balanophyllia (Anthozoa: Scleractinia) in the western Atlantic, with descriptions of four new species. Proceedings of the Biological Society of Washington, 90(1): 132-148.
Cairns, S. D. 1979. The deep-water Scleractinia of the Caribbean Sea and adjacent waters. Studies on the Fauna of Curaçao and other Caribbean Islands, 57: 341 pp.
Cairns, S. D. 1994. Scleractinia of the temperate North Pacific. Smithsonian Contributions to Zoology, 557: 150 pp.
Cairns, S. D. 2001. A Generic Revision and Phylogenetic Analysis of the Dendrophylliidae (Cnidaria: Scleractinia). Smithsonian Contributions to Zoology, 615: 75 pp.
Cairns, S. D., B. W. Hoeksema, and J. van der Land. 1999. Appendix: List of Extant Stony Corals. Atoll Research Bulletin, 459: 13-46.
Cairns, S. D. and S. A. Parker. 1992. Review of the Recent Scleractinia (Stony Corals) of South Australia, Victoria and Tasmania. Records of the South Australian Museum, Monograph Series, 3: 82 pp.
Cairns, S. D. and G. D. Stanley. 1982. Ahermatypic coral banks: living and fossil counterparts. Proceedings of the Fourth International Coral Reef Symposium, Manila, 1: 611-618.
Cairns, S. D. and Zibrowius. 1997. Cnidaria, Anthozoa: Zooxanthellate Scleractinia from the Philippine and Indonesian regions. Mémoires du Muséum National d'Histoire Naturelle, 172: 27-243.
Chevalier, J.-P. 1987. Ordre des Scléractiniares: Systematique. Pp. 679-753 In: Grassé, P. (editor) Traité de Zoologie, 3(3) Masson, Paris.
Grygier, M. J. and W. A. Newman. 1985. Motility and calcareous parts in extant and fossil Acrothoracica (Crustacea: Cirripedia), based primarily upon new species burrowing in the deep-sea scleractinian coral Enallopsammia. Transactions of the San Diego Society of Natural History, 21: 1-22.
Horst, C. J. van der. 1922. The Madreporaria of the Siboga Expedition. Part 3: Eupsammidae. Siboga-Expeditie, 16c: 45-75.
Horst, C. J. van der. 1927. Eupsammid corals from South Africa. Union of South Africa Fisheries and Marine Biological Survey Report No. 5 for the year 1925, Special Report 2: 7 pp.
Marshall, A. T. 1996. Calcification in hermatypic and ahermatypic corals. Science, 271: 637-639.
Romano, S. L. and S. D. Cairns. 2000. Molecular phylogenetic hypotheses for the evolution of scleractinian corals. Bulletin of Marine Science, 67(3): 1043-1068.
Romano, S. L. and S. R. Palumbi. 1996. Evolution of scleractinian corals inferred from molecular systematics. Science, 271: 640-642.
Vaughan, T. W. and J. W. Wells. 1943. Revision of the suborders, families, and genera of the Scleractinia. Geological Society of America, Special Papers, 44: 363 pp.
Veron, J. E. N., D. M. Odorico, C. A. Chen, and D. J. Miller. 1996. Reassessing evolutionary relationships of scleractinian corals. Coral Reefs, 15: 1-9.
Veron, J. E. N and M. Pichon. 1980. Scleractinia of eastern Australia. Part 3. Australian Institute of Marine Sciences, Monograph Series, 4: 422 pp.
Wells, J. W. 1956. Scleractinia. Pp. F328-F444 In: Moore, R. C. (editor) Treatise of Invertebrate Paleontology, Part F: Coelenterata. University of Kansas Press, Lawrence.
Yonge, C. M. 1975. A note on mutualism between sipunculans and scleractinian corals. Proceedings of the International Symposium on the Biology of Sipuncula and Echiura, pp. 305-311.
Zibrowius, H. 1973. Révision des espèces actuelles du genre Enallopsammia Michelotti, 1871, et description de E. marenzelleri, nouvelle espèce bathyale à large distribution: Océan Indien et Atlantique central (Madreporaria, Dendrophylliidae). Beaufortia, 21(276): 37-54.
Zibrowius, H. 1980. Les Scléractiniaires de la Méditeranée et de l'Atlantique nord-oriental. Memoires de l'Institut Océanographique, Monaco, 11: 284 pp.
Zibrowius, H. and M. J. Grygier. 1985. Diversity and range of scleractinian coral hosts of Ascothoracida (Crustacea: maxillopoda). Annals de l'Institut Océanographique, Paris, 61(2): 115-138.
Title Illustrations

| Scientific Name | Turbinaria sp. |
|---|---|
| Location | collected from shallow water off Pulau |
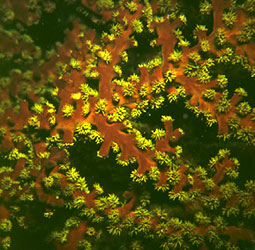
| Comments | A living colony about 1 m in diameter, collected from shallow water off Pulau. This is an attached, colonial dendrophylliid that contains zooxanthellae and contributes to reef structure. |
| Specimen Condition | Live Specimen |
| Size | about 1 m in diameter |
| Copyright | © D. Faulkner |
| Scientific Name | Tubastraea micrantha |
|---|---|
| Location | collected from shallow water off Pulau |
| Comments | A living colony having branches about 20 mm in diameter, collected from shallow water off Pulau. Although azooxanthellate, this species often attains a colony size of up to a meter and thus contributes to reef structure. |
| Specimen Condition | Live Specimen |
| Size | branches about 20 mm in diameter |
| Copyright | © D. Faulkner |
About This Page
Creation of this page was supported by US National Science Foundation grants DEB95-21819 and DEB 99-78106 (in the program PEET - Partnerships to Enhance Expertise in Taxonomy) to Daphne G. Fautin, grant DEB99-78086 (in the program PEET) to Stephen D. Cairns, and grant OCE 00-03970 (in NOPP, the National Oceanographic Partnership Program) to D.G.F. and Robert W. Buddemeier.Technical assistance was rendered by Adorian Ardelean.

Smithsonian Institution, Washington, D. C., USA
Correspondence regarding this page should be directed to Stephen D. Cairns at
Page copyright © 2002
 Page: Tree of Life
Dendrophylliina. Dendrophylliidae .
Authored by
Stephen D. Cairns.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Dendrophylliina. Dendrophylliidae .
Authored by
Stephen D. Cairns.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 28 October 2002
Citing this page:
Cairns, Stephen D. 2002. Dendrophylliina. Dendrophylliidae . Version 28 October 2002. http://tolweb.org/Dendrophylliidae/19165/2002.10.28 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site